Santen is committed to developing new therapies, devices, and other solutions to serve significant unmet needs in vision care and to fuel hope for physicians and patients facing the possibility of vision impairment or loss.
Our US headquarters is an important part of Santen’s global R&D development effort, allowing us to ensure the highest quality standards and an efficient process for developing differentiated ophthalmology solutions.
The following is a summary of therapies currently in development in the United States.
GLAUCOMA
CORNEAL DISORDERS
REFRACTIVE DISORDERS
More About Our Investigational Therapies and Devices
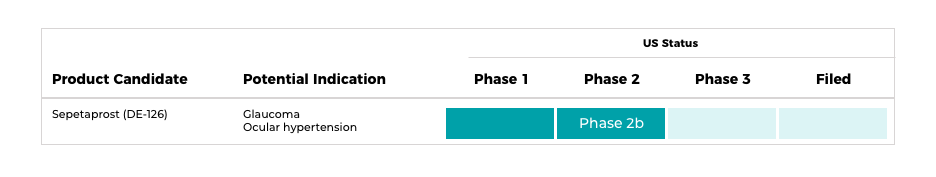
Glaucoma
・Sepetaprost (DE-126)
A prostaglandin eye drop product with a novel mode of action that is both an FP- and EP- receptor dual agonist being developed for the treatment of ocular hypertension and glaucoma. In-licensed from Ono Pharmaceuticals, Inc.
SEE CLINICAL TRIAL >
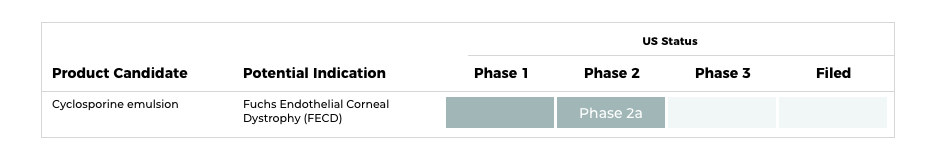
Corneal Disorders
・Ophthalmic suspension
In collaboration with ActualEyes Inc., Santen is currently conducting a clinical trial for the global development of sirolimus eye drops to treat Fuchs endothelial corneal dystrophy (FECD). FECD is a corneal disease that affects approximately 4 percent of people aged 40 or over in the United States and Europe. Characterized by progressive loss of corneal endothelial cells, when FECD advances it can cause scarring or swelling of the cornea resulting in a reduction in or loss of vision. This can also lead to bullous keratopathy, where the cornea becomes permanently swollen. FECD can significantly impact a person’s quality of life.
As the only therapy for treating FECD is the transplantation of a donor cornea, a medication that suppresses the progression of FECD would address an unmet medical need. In addition, sirolimus could provide a novel alternative treatment to a corneal transplant and may reduce the percentage of patients who develop visual impairment. Clinical centers in the United States, France, and India are conducting the trial.
SEE CLINICAL TRIAL >
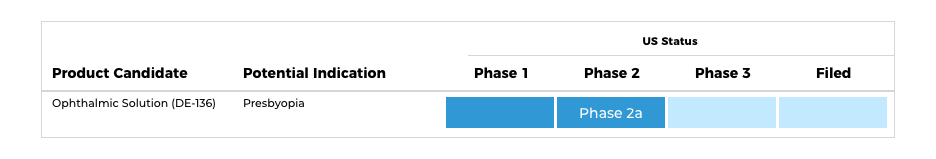
Refractive Disorders
・Ophthalmic Solution (DE-136)
Santen is conducting a clinical trial to assess the efficacy and safety of ursodeoxycholic acid for the improvement of presbyopia by restoring lens elasticity. Clinical centers in the United States and Japan are conducting the trial.
Presbyopia is a refractive disorder that occurs when the clear lens inside the eye becomes more rigid. The lens typically changes shape to focus light onto the retina, allowing the eye to focus on objects that are both close-up and far away.3
SEE CLINICAL TRIAL >
References:
- Moshirfar M, Somani AN, Vaidyanathan U, Patel BC. Fuchs Endothelial Dystrophy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- Bullous Keratopathy. Cornea Research Foundation of America. http://www.cornea.org/Learning-Center/Conditions-Research-Areas/Bullous-Keratopathy.aspx. Accessed July 18, 2022.
- Presbyopia. National Eye Institute. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/presbyopia. Accessed February 27, 2023.
