Production
Production sites that support high quality and stable supply
As a company specialized in ophthalmology, Santen supports the eye health of more than 50 million people in more than 60 countries and regions worldwide. To continue contributing to patients and society, it is our duty to maintain a stable supply of high-quality products.
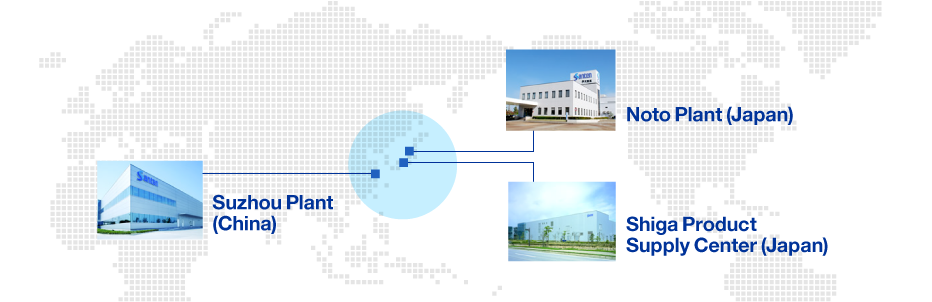
Santen will meet the eye care needs of patients worldwide based on a three-plant system: the Noto Plant, which boasts one of the world’s largest eye drop manufacturing facilities; the Shiga Product Supply Center, a manufacturing base supporting our global growth; and Santen’s only overseas plant, the Suzhou Plant (China), which serves the fast-growing China region.
Quality Commitment and Initiatives
Patients are not always able to visually confirm the quality of a product. Therefore, we believe that ensuring quality in healthcare companies is an important mission that serves as the foundation for the trust earned from patients and healthcare professionals. To ensure the delivery of safe and reliable products, all production processes comply with Good Manufacturing Practice (GMP; standards for the manufacturing and quality control of pharmaceuticals and quasi-drugs), and we are also making various efforts to achieve even higher quality control. Among these, Santen pays particular attention to the “water” used as a raw material, to the “air” on the production line, and to the “people” who manage the production.
Production Process
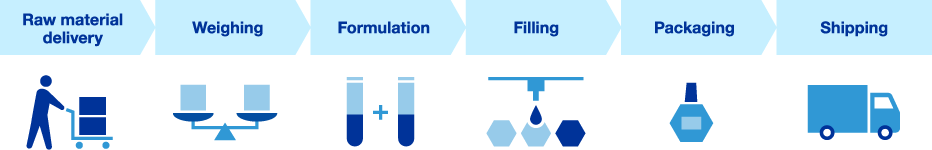
Water
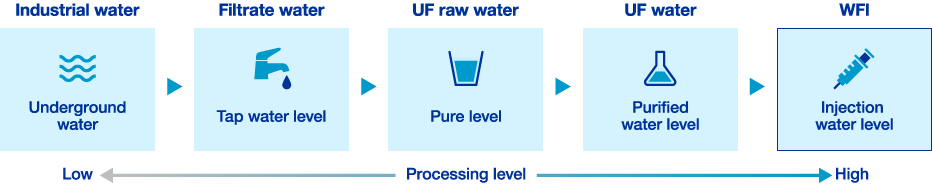
Water, which is vital for ophthalmic solutions, requires high purity to ensure not only safety but also quality. Santen only uses water that has undergone physical treatment, chemical treatment, ion exchange treatment, ultrafiltration*1, distillation, and other purification processes. This applies not only to water for medical eye drops, but also to water used as a raw material for general-use eye drops. Purity is comparable to that of water for injection, which demands particularly strict quality control even under GMP*2. In addition, purified water is used to clean and sterilize pipes and equipment connecting tanks and lines, and we pursue high quality standards for water.
- Water purification method using semipermeable membrane to remove colloidal substances, microorganisms, and macromolecular substances.
- Abbreviation of “Good Manufacturing Practice.” Standards for manufacturing and quality control of pharmaceuticals and quasi-drugs.
Purification Process for Production Use Water
Air
Ophthalmic solutions are defined as “aseptically prepared medicine” in the Japanese Pharmacopoeia*3 in Japan. To maintain sterility, production plants must be very careful to prevent bacteria and other contaminants from entering their production lines and products.
In all of three plants, we conduct risk assessments and ensure thorough air management through appropriate zoning, control of room pressure and airflow, and proper design of flow lines for people and products. More precisely, we maintain a level of cleanliness in compliance with standards by dividing the work environment into specific categories where air cleanliness levels are efficiently designed and adjusted according to contamination risk and constantly monitored.
The weighing and dispensing areas maintain a Grade C*4 air cleanliness level of fewer than 353,000 airborne particles of 0.5 microns or larger per cubic meter when not in operation and fewer than 3.53 million airborne particles per cubic meter when in operation. The filling zone, which requires the strictest attention to bacterial contamination, has achieved and maintains a Grade A4 air cleanliness level, equivalent to a surgical operating room, with fewer than 3,520 airborne particles at all times.
- Specifications and standards for pharmaceuticals, established by the Minister of Health, Labour and Welfare after consultation with the Pharmaceutical Affairs and Food Sanitation Council, to ensure appropriate properties and quality levels for pharmaceutical products in accordance with the Pharmaceutical and Medical Device Act.
- EU-GMP Classification for air cleanliness of sterile pharmaceutical manufacturing spaces
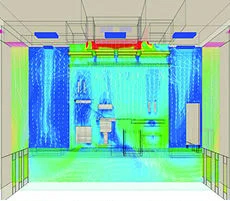
Air flow simulation CG for filling chamber design

Aseptically controlled filling room
People
Employees involved in production and quality assurance support the manufacturing of high-quality products and their stable supply. In pursuit of quality and ease of use from the patient’s perspective, all employees involved in production comply with GMP and receive regular education and training. In addition, by sharing know-how among the three plants and exchanging human resources across regions and departments, we promote collaboration among employees and further enhance their knowledge and skills.

A veteran employee giving technical guidance to team members.

In addition to providing technical guidance, study sessions are regularly held to promote knowledge acquisition and improvement.
Initiatives to Ensure Stable Supply
Santen has established a three-plant system centered around the Noto Plant, Shiga Product Supply Center, and Suzhou Plant (China), and by expanding production capacity to meet diversifying demand, both in Japan and overseas, we are working to build capacity as quickly as possible to ensure stable supply. In addition to rapidly responding to business growth in the global market and the diversification of solutions as we expand into new disease areas, we will strengthen each production base to optimize product lifecycles.
Santen Production Sites
| Plant | Entered Operation | Annual Production Quantity*5 | Special Feature | Products (Examples) |
|---|---|---|---|---|
| Noto Plant | 1985 | 300 million | World's largest eye-drop manufacturing facility | Approx. 60 types of prescription eye drops and 30 types of general use eye drops |
| Shiga Product Supply Center | 1996 | 80-100 million | Supplies products to Japan, Europe, Korea and Asia Responsible for establishing the formulation process | Mainly prescription eye drops, special formulations (steroids, ointments, etc.), clinical trial/diagnostic drugs, etc. |
| Suzhou Plant | 2008 | 32 million | Eye-drop manufacturing facility for the China region | Prescription eye drops |
- Production at our factory. Single-dose containers are aggregated by counting 10 single-dose containers as 1 bottle. All other products are counted based on the actual number of bottles (FY2023).