Safety and Quality Assurance
Santen Safety and Quality Assurance
As a pharmaceutical company, we are obliged to comply with strict control standards for production to ensure that patients can use pharmaceuticals safely and securely.
Quality control at Santen is not limited to production, but covers the entire product life cycle, from research and development to marketing to the patient. By establishing a unique safety and quality assurance system, we have established a foundation for ensuring efficacy, safety, and quality—the three elements that underpin the reliability of pharmaceutical products—on a global level. We are also actively involved in improving patient quality of life and after-sales care, and aim to continue to enhance the reliability of our products and our company.
Under its safety and quality assurance system, Santen has established departments for Quality Assurance (QA), Quality Control (QC), and Safety Vigilance; these departments actively share quality-related information and work together to strengthen safety and quality assurance.
Global Safety and Quality Assurance System Structure
Quality Assurance Initiatives
The Global Quality Management Committee (GQMC), consisting of the General Manager of the Quality Assurance Division and the heads of every function involved in quality assurance, meets regularly to set global policies and standards for quality assurance and auditing.
Safety Monitoring Initiatives
To ensure the safety and peace of mind of patients and their loved ones around the world who use our products, Santen conducts thorough safety monitoring activities and shares information simultaneously across the global organization.
We strive to minimize safety risks to patients through centralized management of safety information collected worldwide via a global database, studying measures required to ensure product safety and proper use, and actively providing information to the medical community.
Quality Assurance (QA)
Assuring quality across the product lifecycle
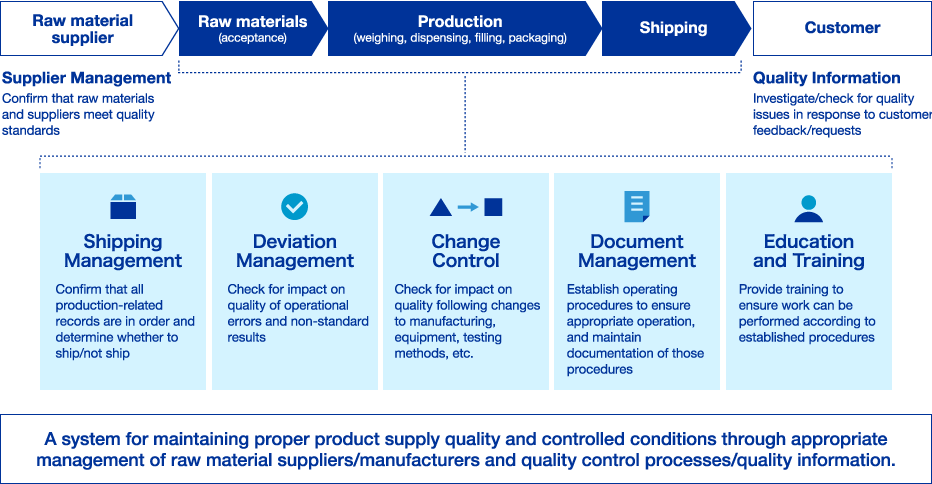
Santen rigorously evaluates and manages all aspects of its pharmaceutical activities, from research and development to production and post-marketing activities, to guarantee the three elements (efficacy, safety, and quality) that underpin the reliability of our pharmaceutical products.
To ensure product reliability, we conduct robust quality control and safety monitoring in collaboration with the research, development, medical affairs, production, and sales departments. Examples of this include confirming that research, non-clinical, and clinical trials are conducted in compliance with regulations, that the quality of raw materials is appropriate, that production line equipment and quality control systems are functioning properly, and that the usage information provided to patients is accurate.
Quality Assurance System
Quality Control (QC)
Control quality within the manufacturing process
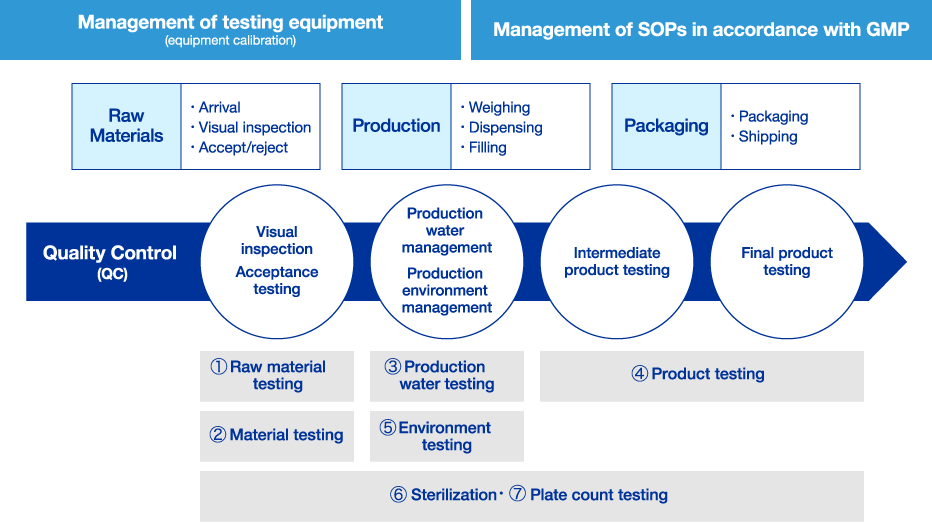
The Quality Control Department is responsible for product quality standards and conducts various tests at each stage of the raw material and production process, including raw material testing, material testing, production water testing, product testing, environmental testing, and sterility/plate count testing. To confirm that quality complies with regulations such as the Pharmaceutical Affairs Law and GMP*1, the department is also responsible for the management of testing equipment, the preliminary inspection of equipment, and the management of equipment procedures.
- Abbreviation of “Good Manufacturing Practice.” Standards for manufacturing and quality control of pharmaceuticals and quasi-drugs.
Quality Control System
To ensure the safety and peace of mind of patients and their loved ones around the world who use our products, Santen conducts thorough safety monitoring activities and shares information simultaneously across the global organization.
Santen strive to minimize safety risks to patients through centralized management of safety information collected worldwide via a global database, studying measures required to ensure product safety and proper use, and actively providing information to the medical community.