Innovation and Execution
We are focused on moving projects as quickly as possible from proof of concept where we have made an initial understanding of the mechanism of action until commercialization. Our understanding of the mechanism then translates itself into a development strategy that takes into consideration, not only the patient voices, but also the market needs. The market needs can be the regulatory environment or the commercial environment, but we believe that all that combined will actually solidify the development strategy. We are also focused on continuing to improve our capabilities with regards to how to maximize the life cycle management of our products. And now, we are looking to further differentiate ourselves. Moving away from just trying to improve the bottle, we also start looking into other disease areas that we have not looked into before. We try to bring out here the maximum for the sake of our patients.
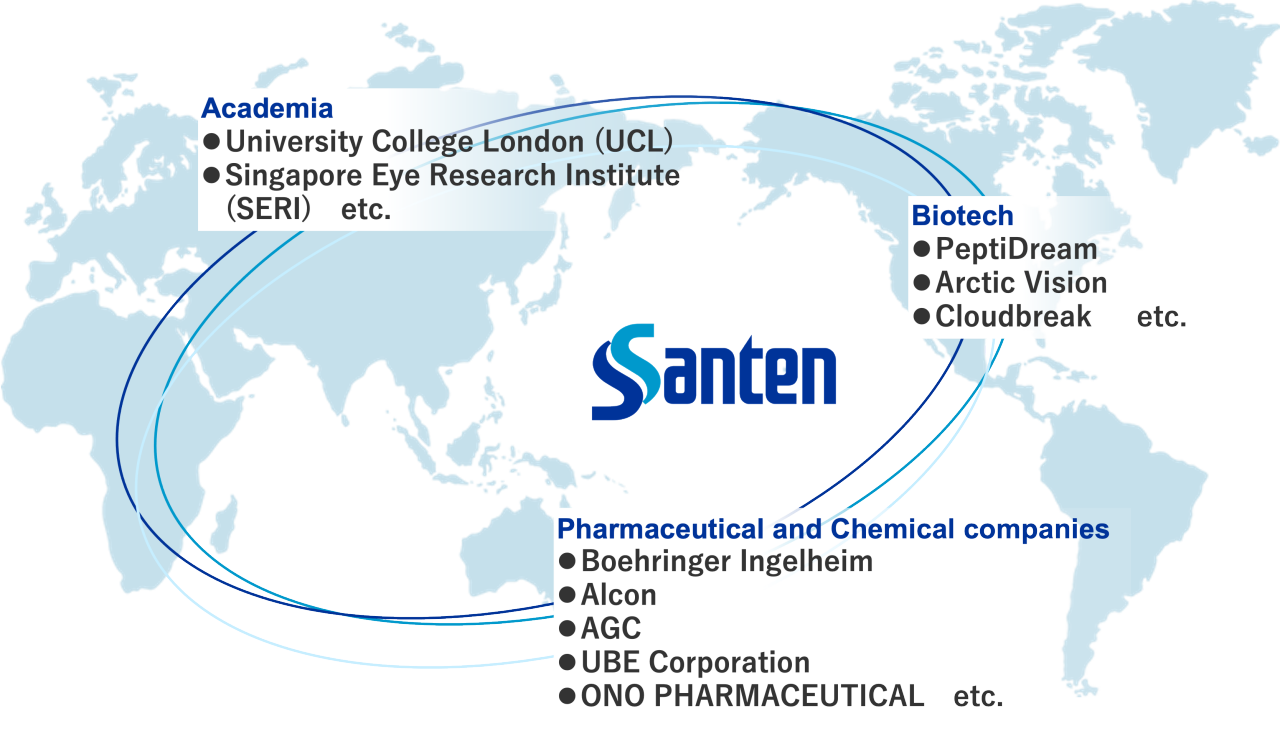
Our global network
Developing new pharmaceuticals entails a long period of over 10 years, as well as a diverse process ranging from basic research to marketing. To free patients from concerns over their illness as soon as possible and deliver happiness through the best vision experience, we must improve the efficiency of our R&D process. To this end, Santen conducts drug discovery and clinical development on a global basis, utilizing facilities of the Santen Group and related organizations worldwide. In this way, we are reinforcing our system to create new products that meet global medical needs in a timely manner. In terms of research activities, within Santen we have concentrated our basic research, non-clinical studies, and drug formulation research at Nara Research and Development Center, so as to integrate the knowledge of our various sections and to work closely together to improve the quality and the speed of research and development, especially in the early stages. We are committed to develop innovative products in collaboration with academia, pharmaceutical and chemical companies, and biotech companies. We believe that building Santen's unique networks is indispensable for continuous product development.
Innovative product development in collaboration with academia, pharmaceutical and chemical companies, and biotech companies
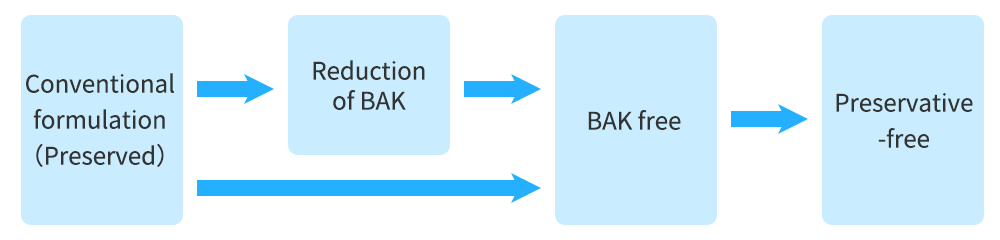
Continuous product improvement for further medication adherence
What we have been good at is taking formulations and moving them from their original state to a more optimal, logical, improved ophthalmic drugs. There are several steps that you can go through in trying to improve products when you are trying to get them to the patients. While benzalkonium chloride (BAK) is used as a preservative, it is considered not to be optimal because it may be associated with unexpected responses, allergy and hyperemia. One of the things that we then did, was to reduce the BAK concentration. Now patients and physicians prefer BAK-free ophthalmic solutions.
This is one of the capabilities we have. The last step is to give patients a preservative-free ophthalmic solution.
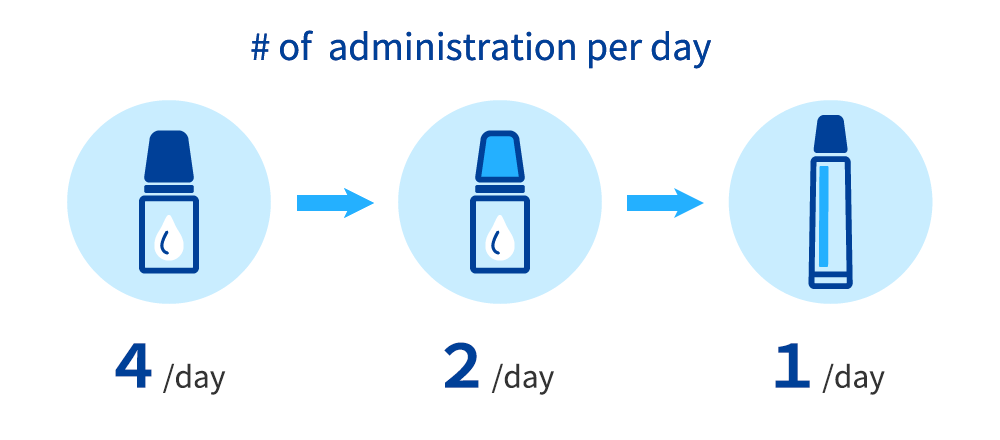
Furthermore, we have been working on improving our existing products. With an aim to improve treatment adherence by reducing dose frequency, we developed a new formulation that reduces frequency of required dosing. In 2024, we made an improvement to the existing twice daily eye drops for treating allergic conjunctivitis and launched the world’s first cream-type product in Japan, requiring just once daily administration to the upper and lower eyelids. Santen hopes that this new product will make it easier for patients to take their required dose, thereby improving patient adherence. The development of the product in a new cream formulation is also expected to help patients who find it difficult to take eyedrops receive their medicine more easily.