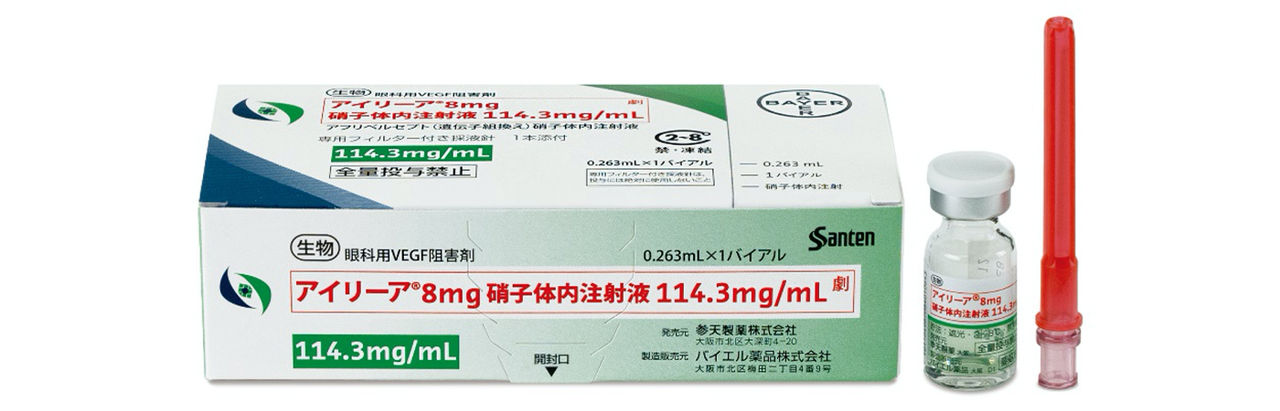
Santen and Bayer Yakuhin Launch Ophthalmic VEGF Inhibitor Eylea®8mg Solution for Intravitreal Injection 114.3 mg/mL
April 17, 2024, Osaka, Japan – Santen Pharmaceutical Co., Ltd. (head office: Osaka, Japan; hereinafter Santen) and Bayer Yakuhin, Ltd. (head office: Osaka, Japan; hereinafter Bayer Yakuhin) today announced the launch of their ophthalmic Vascular Endothelial Growth Factor (VEGF) inhibitor Eylea®8mg Solution for Intravitreal Injection 114.3 mg/mL (aflibercept [recombinant] solution for intravitreal injection; Eylea®8mg).
Ophthalmic VEGF inhibitors are the standard for treatment of many patients with age-related macular degeneration associated with subfoveal choroidal neovascularization (nAMD)1 and diabetic macular edema (DME), both of which may lead to irreversible blindness and vision impairment. These patients require intravitreal dosing with VEGF inhibitors. This unmet need faced by patients who require intravitreal administration of vitreous drugs and medical professionals have long sought the development of a new drug that can extend the dosing interval and reduce the frequency of administration while still mitigating the risk of adverse events related to the administration procedure.
Eylea®8mg is formulated for intravitreal injection at a higher concentration (114.3 mg/mL [8 mg]; injection volume, 0.07 mL) than the previously approved Eylea® solution for intravitreal injection (40 mg/mL [2 mg]; injection volume, 0.05 mL; Eylea®).2, 3 Eylea®8mg is indicated for the treatment of nAMD and DME. In post-induction maintenance treatment of these diseases, Eylea® is usually administered once every two months, whereas Eylea®8mg may usually be administered once every 16 weeks. Realizing sustainable disease control without compromising the efficacy or safety of Eylea® is expected to reduce the burden on patients and extend the dosing interval, making it a new standard of care for these diseases.
In Japan, Eylea®8mg will be distributed by Santen with marketing authorization held by Bayer Yakuhin. Both companies will jointly provide drug information services for Eylea®8mg. Eylea® has six indications, the most for any ophthalmic VEGF inhibitor.2 With Eylea®, Santen and Bayer Yakuhin are helping many patients in Japan improve their visual acuity. Both companies are striving to provide products addressing unmet needs as part of their commitment to further contribute to treatments that enhance the quality of life of patients with retinal diseases.
| Brand name | Eylea®8mg Solution for Intravitreal Injection 114.3 mg/mL |
| Generic name | Aflibercept (recombinant) |
| Indications | Age-related macular degeneration with subfoveal choroidal neovascularization Diabetic macular edema |
| Dosage and Administration | 8 mg (0.07 mL) administered by intravitreal (IVT) injection once every 4 weeks, usually 3 times consecutively (initial phase). The number of consecutive doses may be reduced depending on symptoms. In the subsequent maintenance phase, it is usually administered intravitreally once every 16 weeks. The dosing interval may be adjusted according to the patient’s symptoms, but it should be ≥8 weeks. |
| Date of release | April 17, 2024 |
| National Health Insurance Price | 181,763 yen |
| Date of marketing approval | January 18, 2024 |
| Manufactured and marketed by | Bayer Yakuhin, Ltd. |
| Distributed by | Santen Pharmaceutical Co., Ltd. |
<Product Photograph >
About nAMD and DME
Neovascular (wet) age-related macular degeneration (nAMD) is an eye disease that progresses rapidly and, if left untreated, can lead to vision loss in a few months. nAMD is one of the leading causes of irreversible blindness and vision impairment around the world. nAMD may affect people as they age. It occurs when abnormal blood vessels grow and leak fluid under the macula, the part of the eye deeply involved in controlling central vision to maintain sharp vision. This fluid can damage and scar the macula, thereby causing vision loss. Currently, 196 million people worldwide are living with AMD – it is anticipated that this figure will increase to 288 million by 2040. Approximately 10% to 15% of people with AMD will develop the advanced form nAMD.
DME is a common complication in eyes of people living with diabetes. DME occurs when high levels of blood sugar lead to damaged blood vessels in the eye that leak fluid into the macula. This can lead to vision loss and, in some cases, blindness. Globally, 146 million people are currently living with diabetic retinopathy, which can develop into a more serious condition which is DME. DME is estimated to affect around 21 million people globally.
- Age-related macular degeneration associated with subfoveal choroidal neovascularization is generally known as neovascular age-related macular degeneration (nAMD).
- Eylea® has been approved for the treatment of age-related macular degeneration with subfoveal choroidal neovascularization, macular edema associated with retinal vein occlusion, choroidal neovascularization in pathologic myopia, diabetic macular edema, neovascular glaucoma, and retinopathy of prematurity.
- Concentration (dosage) and volume for indications other than retinopathy of prematurity. For retinopathy of prematurity, aflibercept (recombinant) is administered at a concentration of 40 mg/mL (0.4 mg) and injection volume of 0.01 mL.
Reference
https://www.pharma.bayer.jp/ja/press-release/news2024-03-11
Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial - The Lancet
Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, double-masked, non-inferiority, phase 2/3 trial - The Lancet
About Santen
As a specialized company dedicated to eye health, Santen aspires to contribute to the realization of “Happiness with Vision” by providing products and services to patients, consumers, and medical professionals around the world. Since its establishment, and guided by its CORE PRINCIPLE, “Tenki ni sanyo suru,” Santen has been committed to helping people maintain and improve their eye health for more than 130 years. Santen is engaged in the global research and development, manufacturing, and sales and marketing of pharmaceutical products in the field of eye care, supporting the eye health of approximately 50 million people in more than 60 countries and regions worldwide. Santen’s mission is to provide essential and significant value to patients and society in the prevention, diagnosis, and treatment of eye diseases through products and services created from its expertise in the ophthalmology field and from the patient's perspective. To create a future in which as many patients as possible can lead happy and fulfilling lives, Santen is committed to doing its utmost to realize a society in which people around the world can experience “Happiness with Vision.”
For more information, please visit Santen’s website https://www.santen.com/en.
About Bayer
Bayer is a global enterprise with core competencies in the life science fields of health care and nutrition. In line with its mission, “Health for all, Hunger for none,” the company’s products and services are designed to help people and the planet thrive by supporting efforts to master the major challenges presented by a growing and aging global population. Bayer is committed to driving sustainable development and generating a positive impact with its businesses. At the same time, the Group aims to increase its earning power and create value through innovation and growth. The Bayer brand stands for trust, reliability and quality throughout the world. In fiscal 2023, the Group employed around 100,000 people and had sales of 47.6 billion euros. R&D expenses before special items amounted to 5.8 billion euros. For more information, go to www.bayer.com.
Contact
Corporate Communications
Santen Pharmaceutical Co., Ltd.
Email: communication@santen.com
External Communications, Communications
Bayer Holding Ltd.
Tel: 06-6133-7333