The exterior of the Nara R&D Center, characterized by subdued brown earthen style, a design that reflects Santen’s focus on creating a new attitude toward the future, and curves representing the human eye.
Driven by the desire to serve the unmet needs of patients around the world who are suffering from eye diseases and eye problems, Santen is committed to research and development that promotes "eye health."
Santen is striving to create new pharmaceutical products, and the Nara Research and Development Center (Nara R&D Center) is the nucleus of the Company’s research and development activity in ophthalmology, including diseases in need of new treatments.
We introduce the Nara R&D Center, which continues to promote the eye health of patients and the development of eye care worldwide.
Nara R&D Center at home in one of Japan’s leading “science cities”
The Nara R&D Center was established in 1996 in Ikoma City, Nara Prefecture, one of the leading “science cities” in Japan. The exterior of the building, executed in subdued brown earthen style, harmonizes with the historical area of Nara and represents a desire to “cherish ancient traditions and culture.” Meanwhile, the futuristic glass and aluminum design of the buildings and entrance hall represents “a new attitude toward the future.” The overall design includes a motif expressing the natural curve of the human eye.
The facility currently has approximately 150 scientists and engineers on staff, and about 200 people—including those from the research management office and partner companies—are engaged in research and development activity. To help workers achieve their full potential and promote communication, the building’s interior incorporates a skip-floor structure with wide landing areas, creating an environment in which opinions can be freely and openly exchanged.


Wide landing areas in the skip floor structure promote communication.
A hub for drug discovery research, non-clinical studies & drug formulation research
When the Nara R&D Center was first established, one of its functions was synthesizing and screening compounds as a starting point for in-house drug discovery. Since then, the number of global research and development sites has expanded, and with a focus on returning to Santen’s mission of creating products that meet the world’s diversifying medical needs in a timely manner, the functions of each facility and the global R&D organization have been restructured. The Nara R&D Center currently houses all of Santen’s internal drug discovery research, non-clinical studies, and drug formulation research functions, and performs the various roles required for global and regional product development—including efficacy and safety evaluation, formulation, and product quality design—to high standards in a steady and timely manner.
Contributing to patients and healthcare professionals through pharmacological and safety evaluation and drug formulation development
The main functions of the Nara R&D Center include pharmacological evaluation, which evaluates the pharmacological effects of development candidate compounds and estimates their efficacy and dosage/administration in humans; toxicity/pathological evaluation, which evaluates safety and predicts the risk of adverse drug reactions; and pharmacokinetic evaluation, which confirms the correlation between efficacy and safety information and drug concentration. It also performs important functions in Chemistry, Manufacturing and Controls (CMC: the development of optimal production processes related to the manufacture of pharmaceuticals). During drug formulation development, researchers determine the chemical properties of candidate compounds, examine and verify other ingredients to be included in eye drops, and design eye drops exhibiting excellent quality and safety. In addition, the Nara R&D Center is responsible for setting product standards, developing test methods to ensure quality, developing manufacturing processes to maintain stable commercial production, and transferring technology to manufacturing sites.
To fulfill these functions, the Nara R&D Center includes a laboratory designed to facilitate ophthalmic evaluation, and it is equipped with a variety of specialized instruments. Facilities and equipment are continuously updated to support the focus areas of research and development activities, with the benefits of the expertise and technologies used by scientists and engineers handed down from generation to generation.
For example, in the preparation of pathology specimens—one of several processes used for evaluating safety—Santen is renowned among medical professionals for its advanced techniques for micron-level sectioning and imaging of ocular tissue. In the area of pharmacological evaluation, meanwhile, its ability to conduct rapid efficacy evaluations and generate reliable data has led to many companies approaching Santen to use its compounds and technologies.
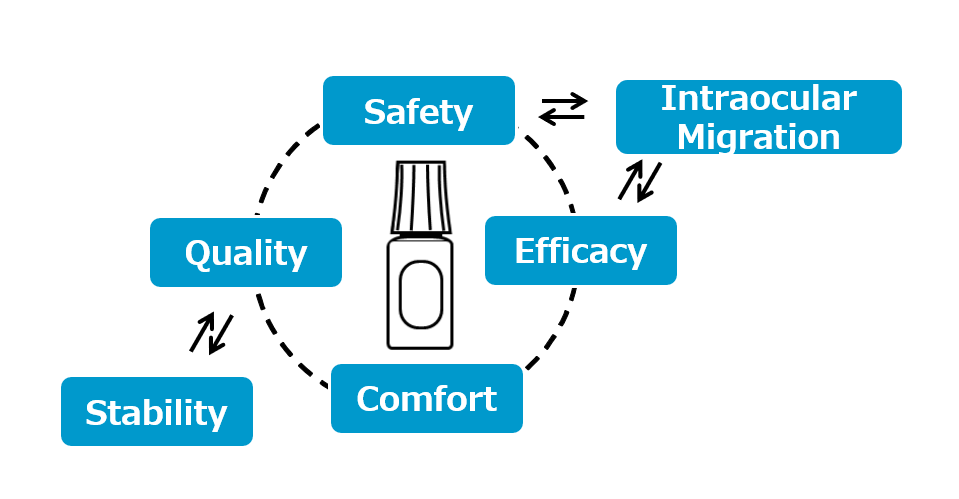
Eye Drop Prescription Design
No “tool” is closer to the eye than the eye drop container, and Santen is committed to making them easy for patients to use
Santen recognizes that eye drop containers are a “tool” that is used close to the eye. For this reason, Santen does not use eye drop containers from external suppliers, but has handled the complete process—from research to manufacturing—in house. To maximize product performance while ensuring patient safety, it is critically important to anticipate how and when patients will be using the eye drops and then successfully align the ingredients and liquid properties of the drug to the material and shape of the eye-drop container. The Nara R&D Center is actively involved in the container development process—from examining the compatibility of the drug and the container material to designing the container itself. The Dimple Bottle, created in 2002, was designed to promote ease of use and visibility for patients who need to apply eye drops every day. It won the Good Design Award in 2008.
- The history of eye-drop bottle evolution
https://www.santen.com/en/business/activity/use
Taking up the challenge of eye disease areas in need of new treatments—supporting eye health for patients worldwide
Santen continues to develop and provide products in its core business areas of glaucoma, dry eye, and allergies, while also pursuing research and development in areas where new treatments are needed, including myopia, presbyopia, and ptosis.
Kazuhito Yamada, who oversees the Nara R&D Center, explains Santen’s future R&D mission and new challenges: “We aim to create new opportunities to contribute value in areas such as myopia, presbyopia and ptosis in the near future. We are also focused on improving access to healthcare. To support the eye health of patients around the world, we will provide new solutions from the Nara R&D Center and contribute to expanding new options for medical care.”

Kazuhito Yamada (second from the right), Head of Pharmaceutics and Pharmacology Department, talks with staffs at Nara R&D Center.
Related pages
- Santen Research and Development
https://www.santen.com/en/business/rd - The evolution of patient-friendly Dimple Bottle eye-drop container
https://www.santen.com/en/stories/20220928
