Santen will contribute to the well-being of society by achieving happy lives through vision around the world, by taking advantage of technology necessary to ensure high quality and safety for products and services which meet the applicable laws, regulations, specifications as well as standards of quality.
Product Quality & Safety
Santen ensures the quality and safety of its products and has an established system for providing products whenever they are needed, to allow patients to use Santen products and services with a feeling of security and trust.
Assurance of Product Life-Cycle Reliability
Quality Principle Policy
The Company seeks to assure the reliability of the product life-cycles of pharmaceuticals according to our "Quality Principle Policy."
To ensure the quality and safety of pharmaceuticals, in accordance with the established training program, all Santen employees involved in production and quality control receive introductory training. This training covers basic knowledge of Good Manufacturing Practice (GMP), production management, quality control, maintenance of manufacturing equipment, and the preparation and management of records. They also receive annual training on GMP.
Quality Assurance Division and Safety Vigilance Department to supervise quality assurance, safety vigilance and auditing with the aim of promoting compliance activities in order to assure pharmaceuticals compliance including product quality, safety and post marketing responses. We also conduct internal audits to ensure the proper operation of the quality management system. Click on the following link to find out more about our assurance of product life cycle reliability.
Measures against Counterfeit Medicines
Santen is working on global supply chain security measures as well as responding to Good Distribution Practice (GDP) in order to protect patients from problems such as health hazards and worsening of medical conditions due to loss of treatment opportunities due to the distribution of counterfeit medicines. We ensure the integrity of our products and operate a lot trace system by implementing features to prevent unauthorized opening of containers and boxes for our products. Also, we are working to strengthen product traceability(*1) by responding to serialization(*2) in accordance with local regulations. In addition, in order to ensure the integrity of legitimate distribution channels, we consider local regulations regarding distribution, assess the risk of distribution channels, evaluate distributors through audits, enter into quality contracts, and monitor defect events.
- Traceability: To be able to track and understand the distribution route from medicine manufacturing to consumption.
- Serialization: Assessing a unique identification code to a product to control distribution from manufacturing to consumption of the medicine
Efforts to Obtain Information Relating to the Safety of Pharmaceuticals
While pharmaceuticals are useful for treatment, they may adversely affect health, for example, due to side effects. For this reason, manufacturers of pharmaceuticals and medical devices in Japan, including Santen, are required by law to report cases of suspected adverse events to the Ministry of Health, Labour and Welfare (MHLW).
Santen has established and documented internal procedures to be taken when we receive information relating to safety, including adverse event information, from patients or healthcare professionals. The procedures require us to report the information immediately and appropriately to our department of safety management, to enable the information to be shared among related departments within the Company. Based on the procedures, we have established a globally effective reliability assurance system, which is also desirable from a pharmacovigilance perspective. We think it is crucial for all of our officers and employees to understand our responsibilities and the actions we should take, so we provide them with adequate training.
Click on the following link to find out more about our Global Quality Compliance.
Product Recall
Our pharmaceutical products are manufactured in accordance with GMP, and any defective products that do not meet specifications are detected and eliminated at each process before delivery. In addition, during the testing processes, products are examined by sampling to ensure quality. If any issues are found regarding the safety, efficacy, quality, labeling or other respects of our products, Santen immediately reports such issues to the authorities, provides the information to medical institutions, and recall applicable products based on our quality assurance system. The number of voluntary product recalls in the past are disclosed in the Social Data.
Prevention of Medical Mistakes
Drug errors may not only prevent patients on such drugs from obtaining the anticipated effect but may also cause the onset of unanticipated side effects. To prevent such medical mistakes, Santen provides clearly identifiable packaging and information labels on containers, in an effort to reduce the workload of medical staff required to identify drugs, as well as to ensure accuracy in handling drugs.
For example, for eye drops available in various concentrations with the same components, we provide information on the concentration on the shrink labels that cover the eye drop containers as well as at the top of the cap in different colors.
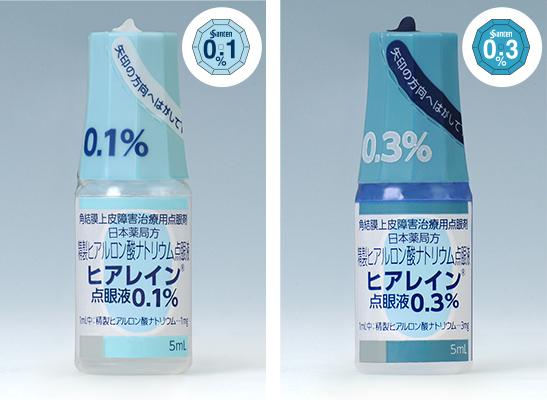 Examples of eye drop containers
Examples of eye drop containers