For healthcare companies, quality assurance is a vital function to ensure the consistent provision of safe and reliable products to patients. This article will provide an overview of the overall strategy of Santen's Quality Assurance Division and review some specific initiatives.
Establishing a solid global quality assurance system
In keeping with our aim of providing people around the world with Happiness with Vision, the Quality Assurance Division is committed to making the existing quality assurance system stronger on a global level, keeping pace with developments such as the diversification of products and services at Santen, our global expansion, and tighter regulations on pharmaceuticals and medical devices in different countries and regions. The Quality Assurance Division has established five strategic pillars for the mid-term plan leading up to 2025, taking into account the various changes that are happening both in the internal and external environment.
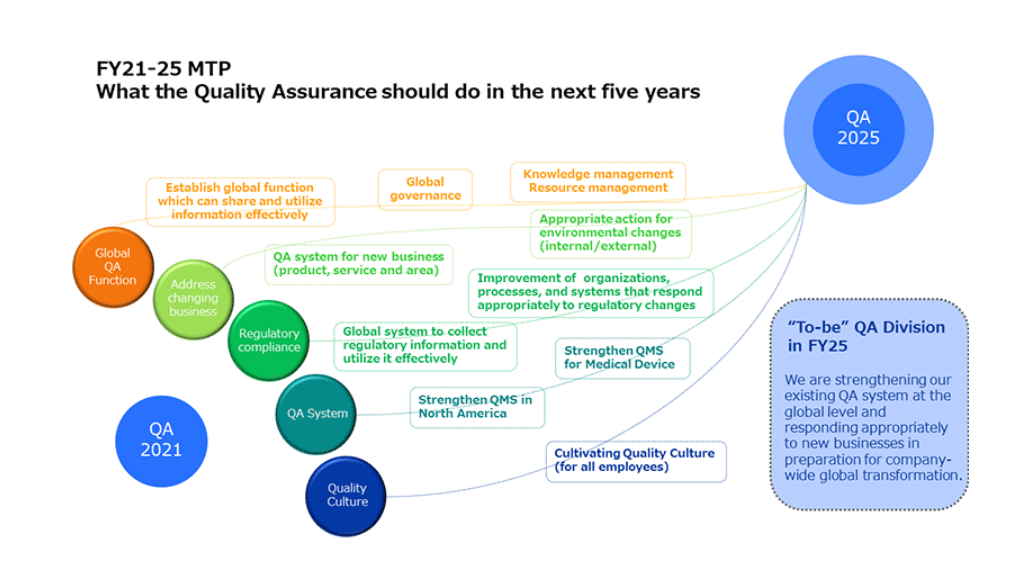
Quality Culture is an important factor supporting Santen's global brand. To ensure a stable supply of safe and reliable products, it is important to cultivate a company-wide organizational culture in which everyone involved in the product—from the research and development stage to the delivery of information to patients and medical institutions and any value we provide following patients’ use of our products—is aware of the need to guarantee and maintain quality. It is also important that the culture encourages each individual to take initiative in ensuring Santen product’s quality. Initiatives to promote this Quality Culture have begun at the Shiga and Noto plants, with plans in the near future to expand the program to include everyone at Santen.
Having everyone participate in developing Quality Culture at the Shiga and Noto plants
The Shiga and Noto plant activities to develop Santen’s Quality Culture were launched in fiscal year 2021. Quality Culture has gained higher attention industry-wide in recent years, triggered by a series of regulatory actions imposed on other pharmaceutical companies for serious regulatory violations. Our plants already had self-checking and other quality assurance initiatives in places, but these were elevated to a new level with the development of a variety of activities to ensure heightened responsibility and engagement for all employees.
The head of the division communicated initial messages to employees, and slogans and goals were set for each factory, team and individual.
Site walk-through events were also held at both factories, where factory members, together with the factory managers and the quality culture administrative office, toured each factory floor. Good Manufacturing Practices (GMP) quiz events were held to further raise awareness, and workshops were held where small, cross functional groups discussed case studies from other companies. These events provided an opportunity for everyone at the plant to think about quality, and to cross functionally exchange opinions on current issues and the future of Santen’s quality assurance.
Through these initiatives, quality culture has become a common topic of conversation across departments, and we have seen an increase in conversations about quality among colleagues even outside of these quality culture events. Workshop participants have noted that the quality culture building events were valuable opportunities for them to speak with their colleagues from other departments.
The site walk-throughs allow participants to take a fresh look at the site by visiting a floor or department different from their own. There are new discoveries every time we organize these events. Rather than making this a top-down activity to identify problems as we walk through the site, we create an atmosphere where people can talk openly with each other about what they have noticed, anything that concerns them, and any problems or errors that have arisen. This makes the walk-throughs an effective opportunity for managers and staff on the ground to come together to discuss quality assurance issues.
We will continue to enhance our quality assurance system, measuring the program’s impact on quality culture, and developing solutions for any issues that are identified.
 The site walk-through
The site walk-through
Messages from members of the Quality Culture administrative office at Shiga and Noto plants.
An Administrative Office member at Shiga commented, “Quality cannot be created or assured by one single department or individual. It is assured by each of our departments fulfilling their roles. I hope these events will encourage everyone to make it a habit to think about quality, and encourage everyone to talk about quality without reservation.”
This was echoed by a Noto Administrative Office member, who said, “Fostering Quality Culture is not something that can be achieved by Quality Assurance’s efforts alone. It is the cumulative result of daily actions by every Santen employee. We will continue to engage in activities that encourage everyone to think about quality.” A colleague added, “We believe that an environment which leads to high quality and stable supply is an environment in which learnings from problems are shared as a way to craft improvements and prevention measures, and in which members are encouraged to proactively participate in the process to produce higher quality products.”
